Relieve annoying cold symptoms
Histolied + Frio Pare
You Will Not Suffer Again
Bedicort plus
Skin repairing agent
Allied Panthenol
For the treatment and prevention of lice
Cidal
Broad spectrum antiseptic action
Allied povidone Vaginal-Gel

About Us
About Us
ALLIED Pharmaceutical Industries is a Syrian private Pharmaceutical company established in 1987 by Dr. Adib shanan & Mr. M.Samir Alsmman, among the creative nature of DARAYA in the south west of Syria. The formulation Plant was inauguration for construction in the year 1994. More than 21 years passed from the first beginning of the company till now and the development is still continuous based on the concept of strength of truth. The plant has its facilities for:
- Product Research & Development.
- Analytical Research & Development.
- Stability testing.
- Quality Control.
- Quality Assurance.
- Manufacturing facility of Antiseptic Liquid, Liquid oral dosages form, Semisolid dosages form, sterile Gauze dressing, MDI and capsules.
- Warehousing.
- Engineering.
-

Dr. Adib shanan
Partner -

Mr. M.Samir Alsmman
Partner
Products
Allied Saline
Sodium chloride

Composition
Sodium chloride 0.9%
Indications
Ideal for daily nasal hygiene. Moisturizes nasal passages to ease breathing. Provides relief from dry nasal passages caused by low humidity in homes, office building, hotels and airplanes.
Sinusal
Oxymetazoline Hydrochloride

Composition
0.05% w\v Oxymetazoline Hydrochloride.
Indications
• Symptomatic treatment of nasal congestion (cold, upper respiratory allergies or hay fever, sinusitis).
• Temporarily relieves sinus congestion and pressure.
Sumet
Metoprolol Succinate

Composition
Each capsule contains extended release pellets of :
23.75 mg Metoprolol Succinate equivalent to 25 mg metoprolol tartrate.
47.5 mg Metoprolol Succinate equivalent to 50 mg metoprolol tartrate.
95 mg Metoprolol Succinate equivalent to 100 mg metoprolol tartrate.
190 mg Metoprolol Succinate equivalent to 200 mg metoprolol tartrate.
Indications
Hypertension:
METOPROLOL XR is indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents.
Angina Pectoris: METOPROLOL XR is indicated in the long-term treatment of angina pectoris.
Heart Failure: METOPROLOL XR is indicated for the treatment of stable, symptomatic (NYHA Class II or III) heart failure of ischemic, hypertensive, or cardiomyopathic origin.
Cardoben
Nicardipine hydrochloride

Composition
Each capsule contains:
Nicardipine hydrochloride 20/30 mg.
Indications
Cardoben dilates blood vessels, which relieves the burden on the heart and lowers pressure
Flucalied plus
Salmeterol Xinafoate + fluticasone propionate
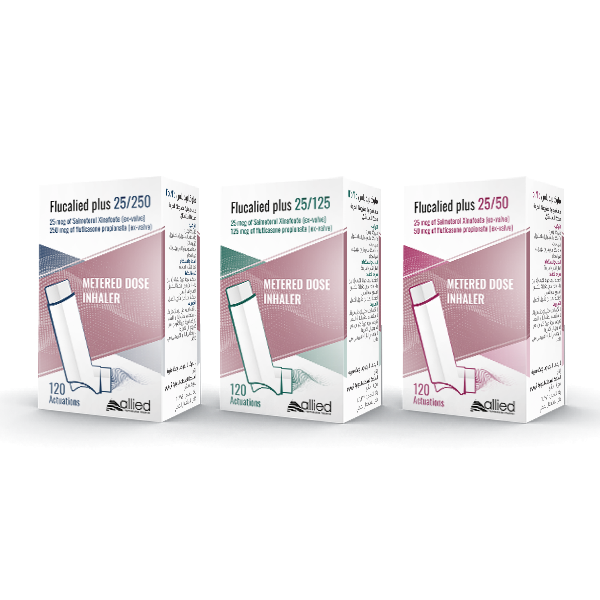
Composition
Each actuation delivers:
25 mcg Salmeterol Xinafoate (ex- valve)+50 mcg of fluticasone propionate (ex-valve).
25 mcg Salmeterol Xinafoate (ex- valve)+250 mcg of fluticasone propionate (ex-valve).
Indications
Salmeterol/ fluticasone is indicated in the regular treatment of asthma where use of a combination product (long-acting β2 agonist and inhaled corticosteroid) is appropriate:
- patients not adequately controlled with inhaled corticosteroids and ‘as needed’ inhaled short- acting β2 agonist or
- patients already adequately controlled on both inhaled corticosteroid and long-acting β2 agonist .
Flutirole
Formoterol Fumarate + Fluticasone Propionate

Composition
Each actuation contains :
6mcg Formoterol Fumarate (ex-valve) and 125/250mcg Fluticasone Propionate.
Excipients: HFA227a, absolute ethanol.
Indications
It is indicated in the regular treatment of asthma, where use of a combination (long-acting beta2-agonist and inhaled corticosteroid) has been found to be appropriate, and in patients with severe COPD.
Allied Salbutamol
salbutamol sulfate

Composition
Each actuation contains 120 mcg salbutamol sulfate (100mcg salbutamol base) (ex-valve) and 108 mcg salbutamol sulfate,(90mcg salbutamol base ) from the mouthpiece.
Indications
Allied salbutamol is indicated for the treatment of prevention of bronchospasm associated with reversible obstructive airway diseases and for the prevention of exercise induced bronchospasm in patients 4 years of age and older.
IPRASMA
Ipratropium bromide

Composition
Each actuation contains 21 micrograms Ipratropium bromide (ex-valve) and 17 mcg of ipratropium bromide from the mouth piece.
Excipients:
HFA134a, Absolute alcohol, anhydrous citric acid.
Indications
IPRASMA is a bronchodilator for maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema.

Heart Disease
Cardoben
The heart pumps blood to carry energy and oxygen around the body A certain degree of pressure in the blood vessels is necessary to complete this process. However, the presence of great pressure in the blood vessels will place an additional burden on the arteries and the heart. This can cause serious complications, such as heart attacks, heart failure, kidney disease, strokes, or dementia.
- Activated Carbon
- Breathing Valve
- 6 Layer Filteration
- Rewashes & Reusable
Latest Products
TIO
Tiotropium Bromide

Composition
Each metered dose contains:
9 mcg Tiotropium Bromide (Monohydrate).
Indications
TIOTROPIUM Inhaler is indicated in the maintenance treatment of COPD.
Bedicort plus
Budesonide + Formoterol

Composition
Each actuation contains:
100/200/400 mcg Budesonide
6 mcg Formoterol (ex-valve).
Indications
FORMOTEROL/BUDESONIDE Inhaler is indicated in the regular treatment of asthma, where the use of a combination (long-acting, beta2-agonist and inhaled corticosteroid) has been found to be appropriate for instance:
Patients not adequately controlled with inhaled corticosteroids and ‘as needed’ inhaled short-acting beta2 adrenoceptor agonists.
Or:
Patients already adequately controlled on both inhaled corticosteroids and long-acting beta2 adrenoceptor agonists.
FORMOTEROL/BUDESONIDE Inhaler is also indicated as maintenance treatment of airflow obstruction in patients with COPD including chronic bronchitis and emphysema.
Flucalied
Fluticasone Propionate

Composition
Each actuation delivers:
125/250 mcg of Fluticasone Propionate
Indications
Fluticasone propionate given by inhalation offers prophylactic treatment for asthma.
Adults:
Mild asthma: Patients requiring intermittent symptomatic bronchodilator asthma medication on a regular daily basis.
Moderate asthma: Patients with unstable or worsening asthma despite prophylactic therapy or bronchodilator alone.
Severe asthma: Patients with severe chronic asthma and those who are dependent on systemic corticosteroids for adequate control of symptoms. On introduction of inhaled fluticasone propionate many of these patients may be able to reduce significantly, or to eliminate, their requirement for oral corticosteroids.
Children:
Any child who requires prophylactic medication, including patients not controlled on currently available prophylactic medication.
BECLOSAB
salbutamol sulphate + beclomethasone dipropionate

Composition
Each dose contains:
120 mcg salbutamol sulphate
(equivalent to 100 mcg salbutamol),
50 mcg beclomethasone dipropionate.
Indications
Treatment of obstructive airway disease, especially intended for patients who require regular doses of both drugs.
IPRATAMOL
ipratropium bromide + salbutamol sulfate

Composition
Each actuation meters:
21 mcg of ipratropium bromide 120 mcg of salbutamol sulfate from the valve and delivers 18 mcg of ipratropium bromide and 103 mcg of salbutamol sulfate (equivalent to 90 mcg salbutamol base) from the mouthpiece.
Indications
IPRATAMOL is indicated for use in patients with chronic obstructive pulmonary disease (COPD) on a regular aerosol bronchodilator who continue to have evidence of bronchospasm and who require a second bronchodilator.
LEVOSAL
levosalbutamol

Composition
Each actuation of the inhaler delivers:
67.8 mcg of levosalbutamol tartrate (equivalent to 51.6 mcg of levosalbutamol free base) from the valve and 59 mcg of levosalbutamol tartrate (equivalent to 45 mcg of levosalbutamol free base) from the actuator mouthpiece.
Indications
Bronchospasm:
LEVOSAL is indicated for the treatment or prevention of bronchospasm in adults, adolescents, and children 4 years of age and older with reversible obstructive airway disease.
SEROLIED
salmeterol

Composition
Each actuation delivers :
25 mcg of salmeterol base (as salmeterol xinafoate) from the valve
21 mcg of salmeterol base (as salmeterol xinafoate) from the actuator.
Indications
Asthma:
Salmeterol Inhalation Aerosol is indicated for long-term, twice-daily (morning and evening) administration in the maintenance treatment of asthma and in the prevention of bronchospasm in patients 12 years of age and older with reversible obstructive airway disease, including patients with symptoms of nocturnal asthma, who require regular treatment with inhaled, short-acting beta2-agonists. It should not be used in patients whose asthma can be managed by occasional use of inhaled, short-acting beta2-agonists.
Salmeterol Inhalation Aerosol may be used alone or in combination with inhaled or systemic corticosteroid therapy.
Salmeterol Inhalation Aerosol is also indicated for prevention of exercise-induced bronchospasm in patients 12 years of age and older.
Chronic Obstructive Pulmonary Disease:
Salmeterol Inhalation Aerosol is indicated for long-term, twice daily (morning and evening) administration in the maintenance treatment of bronchospasm associated with COPD (including emphysema and chronic bronchitis).
Beclolide plus
Beclomethasone dipropionate + formoterol fumarate dihydrate

Composition
Each metered dose (ex-valve) contains:
100 micrograms of Beclomethasone dipropionate and 6 micrograms of formoterol fumarate dihydrate.
Excipients: HFA 134a, anhydrous ethanol, Hcl.
Indications
Beclolide plus is indicated in the regular treatment of asthma where use of a combination product (inhaled corticosteroid and long-acting beta2-agonist) is appropriate:
- patients not adequately controlled with inhaled corticosteroids and ‘as needed’ inhaled rapid-acting beta2-agonist or
- patients already adequately controlled on both inhaled corticosteroids and long-acting beta2- agonists.
This product is indicated in adults.
Allosonide
Ciclesonide

Composition
each actuation delivers 80 / 160 mcg ciclesonide from the mouthpiece.
Indications
Treatment to control persistent asthma in adults and adolescents (12 years and older).
Tio plus
Tiotropium + Formoterol fumarate dihydrate

Composition
Each actuation contains: 9 mcg Tiotropium (as tiotropium bromide monohydrate) + 6mcg Formoterol fumarate dihydrate.
Excipients: HFA134a
Indications
Tio plus is indicated in the maintenance treatment of COPD (chronic obstructive pulmonary disease).
Histolied
phenylephrine + loratadine

Composition
each prolonged release capsule contain:
phenylephrine 20 mg + loratadine 5 mg
as prolonged release pellets.
Indications
Common cold, hay fever (allergic rhinitis) or other upper respiratory allergies
Phenoteledine
Phenylephrine HCl + Triprolidine HCl

Composition
Each capsule contains :
5 mg Phenylephrine HCl
2.5 mg Triprolidine HCl
Excipients: magnesium stearate, Microcrystalline cellulose, sodium starch glycolate.
Indications
Temporarily relieves these symptoms due to common cold, hay fever (allergic rhinitis) or other upper Respiratory allergies:
• Runny nose.
• Sneezing.
• Itching of the nose or throat.
• Itchy, watery eyes.
• Nasal congestion.
• Reduces swelling of nasal passages.
Galado
galantamine (as hydrobromide)

Composition
Each prolonged release capsule contains 8,16,24mg galantamine (as hydrobromide )
excipients: as pellets
Indications
Galantamine is indicated for the symptomatic treatment of mild to moderately severe dementia of the Alzheimer.
Dulo-trans
duloxetine hydrochloride

Composition
Each delayed release capsule contains enteric coated pellets of:
22.4mg duloxetine hydrochloride eq: 20 mg duloxetine.
33.7 mg duloxetine hydrochloride eq: 30 mg duloxetine.
67.3 mg duloxetine hydrochloride eq: 60 mg duloxetine.
Indications
- DULOXETINE is indicated for the treatment of major depressive disorder (MDD).
- DULOXETINE is indicated for the acute treatment of generalized anxiety disorder (GAD).
- DULOXETINE is indicated for the management of neuropathic pain associated with diabetic peripheral neuropathy (DPNP).
- DULOXETINE is indicated for the management of fibromyalgia (FM).
- DULOXETINE is indicated for the management of the Chronic Musculoskeletal Pain.
Fluoxetall
Fluoxetine

Composition
Each capsule contains: 10mg or 20mg Fluoxetine
Excipients: anhydrous colloidal silica, Maize starch, Talc, magnesium stearate.
Indications
Adults:
1- Major depressive episodes.
2- Obsessive-compulsive disorder.
3-Bulimia nervosa: Fluoxetine is indicated as a complement of psychotherapy for the reduction of binge-eating and purging activity.
Children and Adolescents Aged 8 Years and Above:
Moderate to severe major depressive episode, if depression is unresponsive to psychological therapy after 4-6 sessions, antidepressant medication should be offered to a child or young person with moderate to severe depression only in combination with a concurrent psychological therapy.
Z-Aqua Cream
Zinc oxide

Composition
Zinc oxide11.3g , mineral oil, beeswax, microcrystalline wax, synthetic beeswax, fragrance, sorbitan sesquioleate, oenothera biennis (evening primrose) seed extract, olea europaea (olive) leaf extract, dimethicone, tocopherol, methylparaben, glycine soja (soybean) oil, propylparaben, panthenol, sarcosine, benzoic acid, potassium aspartate, potassium hydroxide, magnesium aspartate, sodium cocoyl amino acids, water.
Indications
Z-Aqua cream helps:
• helps relieve and prevent rashes and irritation due to wetness from incontinence.
• helps to relief chafed skin resulting from irritation and helps seal out moisture of the skin.
CIDAL
Permethrin 1%

Composition
texapon, cremophor RH40, perfume, water
Indications
Cidal preparations are used for the cases of infestation
with Sarcoptes scabies (scabies)and lice.
Betamethasone Allied
betamethasone valerate

Composition
Each gram of the Cream contains 1.2 mg betamethasone valerate (equivalent to 1 mg Betamethasone)
Indications
Betamethasone Allied is a potent topical corticosteroid indicated for adults, elderly and children over 1 year for the relief of the inflammatory and pruritic manifestations of steroid responsive dermatoses. These include the following:
• Atopic dermatitis (including infantile atopic dermatitis).
• Nummular dermatitis (discoid eczema).
• Prurigo nodularis .
• Psoriasis (excluding widespread plaque psoriasis).
• Lichen simplex chronicus (neurodermatitis) and lichen planus.
• Seborrhoeic dermatitis.
• Irritant or allergic contact dermatitis.
• Discoid lupus erythematosus .
• Adjunct to systemic steroid therapy in generalized erythroderma.
• Insect bite reactions.
Alliedvil
Chlorpheniramine Maleate, Ibuprofen, Phenylephrine Hydrochloride

Composition
Each capsule contains Chlorpheniramine Maleate 4mg, Ibuprofen 200mg,
Phenylephrine Hydrochloride 10mg.
Indications
• Temporarily relieves these symptoms associated with hay fever or other upper respiratory allergies, and the common cold:
• Runny nose, itchy, watery eyes, itching of the nose or throat.
• Sneezing, nasal congestion, sinus pressure.
• Headache, fever, minor body aches and pains.
• Reduces swelling of the nasal passages.
• Temporarily restores freer breathing through the nose.
Apremand
Aprepitant

Composition
One capsule (pellets) contains: 125mg Aprepitant.
Two capsules (pellets) contains: 80 mg Aprepitant.
Indications
1. Prevention of Chemotherapy Induced Nausea and Vomiting (CINV): the capsules, in combination with other antiemetic agents, are indicated in patients 12 years of age and older for the prevention of:
• Acute and delayed nausea and vomiting associated with initial and repeat courses of highly emetogenic cancer chemotherapy (HEC) including high-dose cisplatin.
• Nausea and vomiting associated with initial and repeat courses of moderately emetogenic cancer chemotherapy (MEC).
2. Prevention of Postoperative Nausea and Vomiting (PONV) in adults
Bedicort
Budesonide

Composition
Each spray contains budesonide 64 micrograms (1.28 mg/ml).
Excipients: Dispersible cellulose (Microcrystalline cellulose and carboxymethylcellulose sodium, Polysorbate 80 , Potassium sorbate, dextrose, anhydrous, Disodium edetate, Hydrochloric acid, Ascorbic acid, Purified water.
Indications
Seasonal and perennial allergic rhinitis and vasomotor rhinitis. Treatment of nasal polyps.
Clotritopic
Clotrimazole

Composition
Each 1 g of cream contains:
10 mg clotrimazole.
Indications
clotrimazole Cream is used to treat fungal skin infections such as ringworm, athlete’s foot, fungal nappy rash and fungal sweat rash. It is also used to relieve irritation of the vulva (external thrush) or the end of the penis, which may be associated with thrush.
Dapotopic
Dapsone

Composition
Each 1g Gel Contains 75 mg Dapsone.
Excipients: Diethylene Glycol Monoethyl Ether, Methylparaben, Polysorbate 80, Acrylamide, Isohexadecane, Purified Water.
Indications
It is used for topical treatment of acne vulgaris.
Flucalied
Fluticasone propionate

Composition
Each metered dose contains 50 micrograms of fluticasone propionate.
Excipients: 0.02% w/w benzalkonium chloride, dextrose, microcrystalline cellulose and carboxymethylcellulose sodium, 0.25% w/w phenylethyl alcohol and polysorbate 80.
Indications
The prophylaxis and treatment of seasonal allergic rhinitis (including hay fever) and perennial rhinitis. Fluticasone propionate has potent anti-inflammatory activity but when used topically on the nasal mucosa has no detectable systemic activity.
Minoxidil Shampoo
Minoxidil

Composition
Minoxidil 5%, sodium laurel sulfate, Diethanolamine DEA, Coconut Oil, Glycerin, hydroxyethyl cellulose, Propylene Glycol, Provitamin B5, Vitamin B6, EDTA, Palmitate Vitamin A, Deionized water
Indications
A shampoo that helps increase hair growth, in addition to containing vitamin B6, it helps maintain healthy skin and scalp, thus promoting better hair growth.
Xylollied
xylometazoline hydrochloride
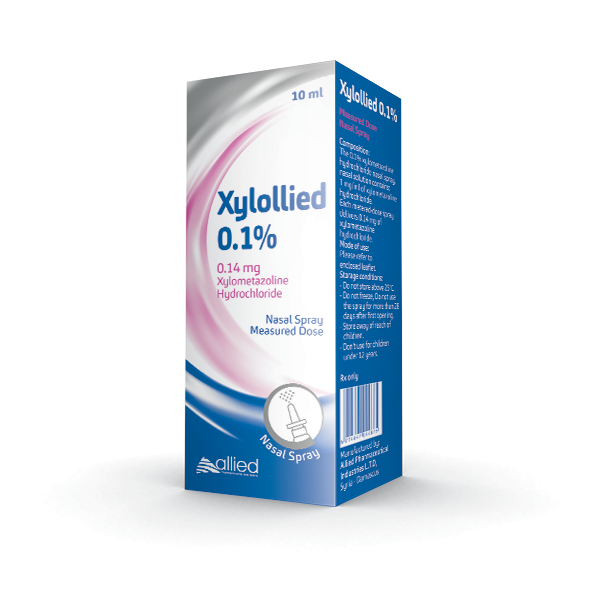
Composition
The 0.1% xylometazoline hydrochloride Nasal Spray, nasal solution contains 1 mg/ml of xylometazoline hydrochloride. Each metered-dose spray delivers 0.14 mg of xylometazoline hydrochloride.
excipients: sodium chloride, Disodium phosphate dodecahydrate (Sodium phosphate ) , Sodium dihydrogen phosphate dihydrate (Sodium acid phosphate), disodium edetate, benzalkonium chloride and water .
Indications
For the symptomatic relief of nasal congestion, perennial and allergic rhinitis (including hay fever), sinusitis.
























